
Aliquoting FBS for Cell Culture:
Best Practices, Risks, and Alternatives

Aliquoting FBS — fetal bovine serum — is one of the most common practices in cell culture labs. It provides crucial nutrients and growth factors that support cell growth and proliferation, typically added at a final concentration of 10%.
Most researchers aliquot their FBS from larger bottles into 50‑ml conical tubes and refreeze them for later use, ensuring that only the required amount is thawed when needed.
This well-known process helps avoid unnecessary freeze/thaw cycles and may seem flawless, yet it comes with several hidden risks that can impact research quality.
In this article, we explore the potential problems of aliquoting FBS and share best practices to minimize variability and contamination risks.
Risks & Problems of Aliquoting FBS
RISK OF CONTAMINATION
Aliquoting FBS increases the chance of microbial contamination, especially with an improper aseptic technique. Contaminants can severely impact cell growth and behavior, leading to false experimental results.
FREEZE/THAW CYCLES
FBS usually undergoes numerous freeze/thaw cycles before being added to the culture medium. Repeated freeze/thaw cycles can lead to the degradation of proteins and diminish the overall quality.
COST & RESOURCES
Aliquoting FBS can be very tiresome and time-consuming, while using additional resources. Moreover, labeling errors and improper inventory management can further increase the costs.
Quick Links
Risks & problems of refilling large bottles of FBS into smaller aliquots.
Take a look at our FBS Minis: the time-saving and risk-free solution.
Contamination During Aliquoting of FBS
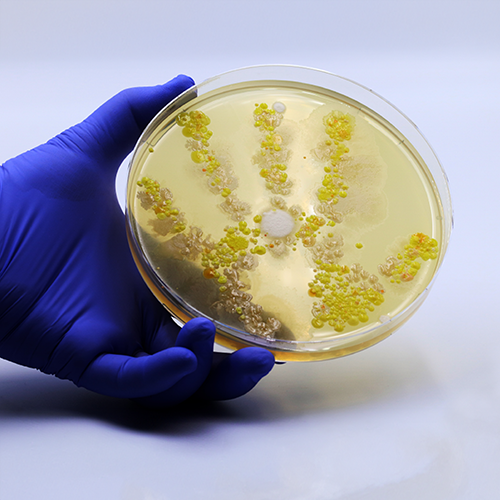
While dividing FBS into smaller aliquots, there is an increased chance of microbial contamination, such as bacteria, fungi, and viruses. This is true especially if aseptic techniques are not followed diligently.
The Consequence? Unreliable Results for Your Experiments!
The contamination is not always detected (also many laboratories add antibiotics to their culture medium by default), which can severely impact cell growth and behavior, leading to unreliable experimental results.
Avoiding Contamination: Come Prepared!
Handling cell cultures is always a meticulous endeavor, requiring foresight and a structured approach. The same applies to aliquoting FBS.
The following provides useful considerations and guidelines for contamination-free FBS aliquoting:
1. Prepare Workspace, Materials & PPE:
- run laminar flow hood for at least 15–30 minutes before starting to ensure a sterile environment
- clean the laminar flow hood by wiping down all surfaces with 70% ethanol
- gather conical tubes, pipette, and permanent marker
- wear gloves, lab coat, and any other required protective gear
2. Disinfect Materials & Position Items Within Reach:
- disinfect all materials and equipment that will be used in the laminar flow hood
- wipe down the pipette (if applicable), conical tubes, and any other tools with 70% ethanol
- arrange materials in strategic manner within hood, ensuring everything is easily accessible
- minimize unnecessary movements to reduce the risk of introducing contaminants
3. Follow Aseptic Technique:
- wash your hands thoroughly before starting
- avoid unnecessary talking or movements during the procedure
- close each tube promptly to prevent airborne contaminants from entering
4. Label Conical Tubes:
- clearly label each conical tube with the necessary information
- include your name, date, batch number, or any other relevant details
By following these meticulous steps and maintaining a sterile environment, you can significantly reduce the risk of contamination during the FBS aliquoting process.
Additionally, periodic testing of FBS batches for microbial contamination can help ensure the quality of the starting material. Make sure to choose a reputable manufacturer with a well-established and documented quality management system (QMS) for your serum products.
Freeze/Thaw Cycles

It is not uncommon that FBS will undergo numerous freeze/thaw cycles before being used for a cell culture experiment. Often times, the shipment arrives in the lab, where it may partially thaw if not moved to the freezer directly. On aliquotation day, it is thawed again and aliquoted into conical tubes. Finally – you guessed it – it is refrozen and thawed on the day of addition to the culture medium.
As you can see, there can potentially be many freeze/thaw cycles, each of which may affect the stability and activity of growth factors and other essential components in the serum. Therefore, repeated freeze-thaw cycles can lead to the degradation of proteins and diminish the overall quality of FBS – once again, tampering with the reliability and reproducibility of your experimental results.
To mitigate these issues, there are some simple guidelines you can follow:
1. Handle FBS with Care After Delivery
- ensure FBS is promptly moved to freezer upon arrival to minimize partial thawing
- alternatively, plan ahead and begin the aliquoting process immediately
2. Prepare Aliquots According to Usage
- if you supplement your culture medium with 10% FBS, prepare 50-ml aliquots
- speak with your coworkers and plan their usage as well
3. Store Aliquoted FBS Properly
- store aliquots at a consistent and appropriate temperature to minimize fluctuations
By taking these precautions, you maintain the stability and functionality of FBS, ensuring its optimal performance and promoting more reliable and reproducible results.
Uneven Distribution of Key Components

Most people are not aware of this issue, yet nutrient gradients can form within a single bottle of FBS. Hence, aliquoting into smaller vials may lead to uneven distribution of growth factors and nutrients, resulting in fluctuating performance across experiments.
Inconsistent and unreproducible research data hinder your ability to draw conclusions from the time-consuming data you have obtained! Consequently, when aliquoting FBS, it is imperative to fully thaw and mix the entire bottle before commencing.
Furthermore, when aliquoting manually, it is not uncommon to make pipetting mistakes that result in volume discrepancies. Some aliquots may get more volume than others. While your cells will grow either way, the way they respond in terms of growth rate, cell health, and metabolism can vary.
Improper Inventory Management

Like all biological products, FBS has a finite shelf life. Even when aliquoted and stored properly, FBS can deteriorate over time due to factors like temperature fluctuations and exposure to light. As FBS ages, the levels of essential nutrients and growth factors may decline, impacting cell culture performance.
Unfortunately, it is not uncommon that the 50-ml aliquots with FBS get lost in the black hole of the lab freezer. Even if lost and found again, it is possible that the product has deteriorated and will no longer perform as expected. Additionally, the labels on these aliquots may become unreadable due to smudging, further complicating the identification and usability of the samples.
To ensure the quality of FBS, it is essential to monitor the expiration date and avoid using expired or outdated aliquots. Implement a strict first-in-first-out (FIFO) system to utilize older aliquots before newer ones. Additionally, use a permanent marker or smudge-proof labels when aliquoting to guarantee that vital information, including expiration dates, remains legible, preventing any unintentional use of outdated samples in cell culture experiments.
Conclusion

Aliquoting fetal bovine serum for cell culture remains a common practice in research laboratories worldwide.
However, it is essential to be aware of the aforementioned risks associated with this process and take appropriate measures to mitigate them. The outcome of your experimental results depends on it!
By following proper aseptic techniques, characterizing FBS lots, minimizing freeze-thaw cycles, and managing resources efficiently, you can enhance the reliability and reproducibility of your research, leading to more accurate scientific discoveries and advancements.
Frequently Asked Questions (FAQs)
When should I aliquot my FBS?


You should aliquot your FBS directly upon receipt. This practice is essential to minimize the number of freeze/thaw cycles that fetal bovine serum (FBS) undergoes, ensuring its stability and preserving its quality.
By dividing the serum into smaller, single-use portions immediately, you reduce the risk of contamination and maintain consistency in experimental results.
Aliquoting is particularly crucial for long-term storage, allowing you to thaw only the needed amount for specific experiments and minimizing waste. This approach not only safeguards the integrity of the FBS, but also contributes to the overall success and reliability of your cell culture work.
Do I have to aliquot FBS?


Yes, aliquoting fetal bovine serum (FBS) is strongly recommended. This practice helps preserve the quality of the serum by minimizing freeze-thaw cycles, reducing the risk of contamination, and ensuring consistency in experimental results. It is a crucial step for maintaining the stability and reliability of FBS in cell culture applications.
Additionally, for a more convenient and care-free solution, consider our FBS Minis. These are pre-aliquoted FBS bottles that not only save you time and effort but also come with many other benefits. Enjoy a hassle-free approach to ensure the integrity of your FBS and reproducibility of your experimental results!
What is the recommended vial or container for aliquoting FBS?


Typically, sterile 50-ml conical tubes are used for aliquoting. However, you may use a variety of sterile, sealable reaction tubes suitable for your specific application. These containers should have secure sealing capabilities to prevent contamination and minimize the risk of freezer burn.
Ensure that the vials are labeled with essential information such as date, batch number, and any other relevant details for traceability. Using suitable containers is crucial to maintain the integrity of FBS during storage and handling.
Additionally, consider our FBS Minis as an ideal solution. These pre-aliquoted FBS vials come in orderly 50-ml bottles, providing a convenient and efficient way to handle FBS. With FBS Minis, you eliminate the need for manual aliquoting, ensuring accurate volumes and minimizing the risk of contamination.
What do I have to keep in mind when aliquoting FBS?


When aliquoting, prioritize sterile technique to prevent contamination. Utilize a clean workspace, appropriate personal protective equipment, and work in a controlled environment such as a laminar flow hood. Choose suitable sterile, sealable reaction tubes for aliquoting. Clearly label each aliquot with essential information for traceability and to prevent confusion during experiments.
Additionally, minimize freeze/thaw cycles by aliquoting into smaller volumes, store samples at recommended temperatures, and use separate tools for each aliquoting step to prevent cross-contamination.
Alternatively, you could consider the convenience of pre-aliquoted solutions, such as our FBS Minis, which eliminate manual aliquoting, ensuring accurate volumes and minimizing contamination risks!
What is the recommended volume for FBS aliquots?


The volume of Fetal Bovine Serum (FBS) for each aliquot depends on the final concentration you intend to use in your cell culture medium.
We suggest creating the aliquots in a way that allows you to use each as a single-use aliquot for one bottle of cell culture medium. This practice not only ensures convenient and precise usage but also minimizes the need for multiple freeze/thaw cycles, preserving the quality of the FBS throughout your cell culture experiments.
Many researchers find that aliquoting FBS into volumes of 50 ml is a common and practical approach. Consider your specific experimental needs and the volume required for a single batch of cell culture medium when determining the ideal aliquot size for your FBS.
For added convenience, consider exploring our FBS Minis. These pre-aliquoted FBS vials provide a ready-to-use solution, eliminating the need for manual aliquoting.
Is it recommended to use a pipette when aliquoting, or can I pour the FBS into aliquots?


It is highly recommended to use a pipette when aliquoting fetal bovine serum (FBS). Using a pipette allows for precise measurement and controlled dispensing of the serum, ensuring accurate volumes for each aliquot.
This precision is crucial for maintaining consistency in cell culture experiments and preventing variations in FBS concentrations. Pouring FBS directly may lead to inaccuracies and uneven distribution, potentially affecting the quality of your samples.
If you are looking to avoid manual aliquoting, consider purchasing our pre-aliquoted FBS Minis. The serum is carefully measured and sealed in sterile conditions, ensuring accuracy and even distribution across each 50-ml bottle.
How should I thaw my FBS aliquots?


We recommend thawing fetal bovine serum (FBS) aliquots gradually by transferring them from the freezer to a refrigerator (4 °C). Avoid rapid thawing methods like microwaving or water baths, as these can compromise the quality of the serum.
After thawing, gently mix the FBS by inverting the vial to ensure homogeneity.
Did you know? Using our pre-aliquoted FBS Minis, the bottles can be thawed within 6 hours in the refrigerator, providing a faster turnaround time for your cell culture needs!
What information should I label on my FBS aliquots?


When labeling your fetal bovine serum (FBS) aliquots, consider the following key details for traceability and experimental organization:
- Date of Aliquoting: Clearly mark the date when the FBS aliquots are prepared, to monitor their age and ensure timely usage.
- Batch Number: Assign a unique batch number to each aliquot for easy tracking and identification.
- Experiment-Specific Details: Include any experiment-specific information relevant to your workflow, such as project name, researcher name, or other experimental notes.
Ensure that your labeling is clear and accurate (using permanent marker) to prevent any confusion during experiments and to maintain the integrity of your FBS supply.
Want the fail-proof method?
Our FBS Minis offer an additional level of convenience in labeling. They come in practical folding boxes that provide ample space for detailed information.
On the box, you can write important details such as your name, lab group, specific notes, and even keep track of the remaining bottle count!
The box and each bottle inside the box are also labeled with essential information, including product name, lot number, expiry date, and more. This meticulous labeling system on both the box and individual bottles ensures efficient organization and easy retrieval of information when using FBS Minis in your experiments.
Instructions for Use
How to properly thaw fetal bovine serum (FBS)


Thawing fetal bovine serum (FBS) is a critical step to ensure its optimal performance in cell culture. Follow these guidelines for the best results:
- Gradual Thawing: Transfer the FBS aliquot from the freezer to a refrigerator (4°C) for gradual thawing. Allow the serum to thaw slowly overnight or for a minimum of 6 hours.
- Avoid Rapid Thawing: Refrain from using methods like microwaving or water baths for quick thawing, as they can compromise the quality of the serum.
- Gentle Mixing: After thawing, gently mix the FBS by inverting the vial several times to ensure a homogenous solution. Avoid vigorous shaking to prevent foaming.
Did you know? Thawing our FBS Minis is super convenient. These pre-aliquoted FBS vials can be thawed within 6 hours in the refrigerator, providing a faster turnaround time for your cell culture needs. The same gradual thawing process is recommended for FBS Minis to ensure optimal performance.
How to minimize contamination risks while aliquoting FBS


Contamination risks during fetal bovine serum (FBS) aliquoting can be mitigated with careful practices.
Firstly, ensure a sterile workspace and use proper personal protective equipment. Work in a laminar flow hood to maintain a controlled environment. Choose sterile containers and equipment, and employ aseptic techniques throughout the aliquoting process. Additionally, minimize the duration of the aliquoting process to reduce exposure.
Want a risk-free option?
Our FBS Minis offer an additional layer of protection against contamination. These pre-aliquoted 50-ml FBS bottles are filled under strict cleanroom conditions in a qualified process. The sterile conditions during manufacturing ensure that the FBS Minis are free from contaminants, providing a convenient and reliable solution for your cell culture needs.
Use our FBS Minis to streamline your workflow and minimize the risk of contamination during aliquoting!
How to properly label FBS aliquots


Properly labeling fetal bovine serum (FBS) aliquots is crucial for maintaining organization and traceability. Include key details such as the date of aliquoting, a unique batch number, and experiment-specific information like project name or researcher details. Clear and accurate labeling helps prevent confusion and ensures the integrity of your FBS supply during cell culture experiments.
Consider using our FBS Minis for added convenience in labeling. These pre-aliquoted FBS vials come with a practical folding box that allows you to write essential information like your name, lab group, notes, and keep track of the current bottle count.
Each bottle inside the box is individually labeled with important details such as product name, lot number, and expiry date.
This meticulous labeling system on both the box and individual bottles enhances organization and facilitates easy retrieval of information for your experiments.
How to save time when aliquoting FBS


Saving time during Fetal Bovine Serum (FBS) aliquoting can be achieved through efficient practices. Firstly, consider using pre-aliquoted solutions like our FBS Minis. These vials are filled under strict cleanroom conditions in a qualified process, eliminating the need for manual aliquoting. With FBS Minis, you save time on preparation and eliminate the risk of contamination, streamlining your cell culture workflow.
Additionally, organize your aliquoting process by setting up a dedicated workspace (laminar flow hood) with all necessary tools and materials readily available and in hand's reach. Properly plan and label aliquots in batches to minimize interruptions and maintain a smooth workflow. By adopting these time-saving strategies, you enhance efficiency and optimize your FBS aliquoting process.
FBS Minis
Less Work. More Time.
Designed to streamline your cell culture workflow, FBS Minis provide a hassle-free alternative to the traditional process of aliquoting.
Filled directly into practical 50-ml bottles, the FBS Minis eliminate the need for manual handling and thereby also the risk of contamination and errors. Not only do they save time, but they also ensure precise and consistent aliquots, minimizing the potential for fluctuations in nutrients and growth factors!
Choosing FBS Minis is a straightforward process. Start by testing any of our serum products as you normally would. Once you are convinced of the quality and performance, you can conveniently order the same serum as FBS Minis filling option.
This allows you to seamlessly transition to a more efficient and reliable aliquoting solution, ensuring uniform distribution and maintaining the integrity of your cell culture experiments.